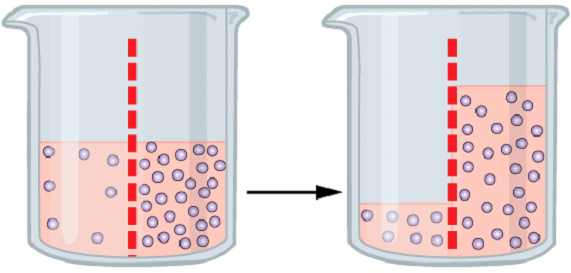
Diffusion and osmosis work in the same way. Where diffusion works in an open space, osmosis works between two solutions that are separated by a semi-permeable membrane. In essence, the movement of molecules from an area of higher concentration to an area of lower concentration is called diffusion.
So, what is Osmosis?
Osmosis is the phenomenon in which no energy is required. It occurs in two different solutions where the concentration of solute varies. The movement of solute molecules from the region of higher concentration to the region of lower concentration through a semi-permeable membrane until both sides have an equal amount is called osmosis.
There are three different types of osmotic solutions. Osmosis itself mostly occurs in cells. There are two types of osmosis, so let’s learn about them!
Types of Osmotic Solutions
The three different types of osmotic solutions found in science are:
⦁ Hypertonic Solution
When the solute concentration is higher outside the cell as compared to the inside, the solution is called a hypertonic solution.
⦁ Hypotonic Solution
When the solute concentration is higher on the inside of the cell as compared to the outside, the solution is called hypotonic.
⦁ Isotonic Solution
When the concentration of solute particles is equal on both sides of the cell, the inside as well as the outside, it is called an isotonic solution.
Different Types of Osmosis
In the biological world, there are two types of osmosis:
⦁ Endosmosis
When a cell is put in a hypotonic solution, the solute concentration is higher on the inside of the cell; therefore, the solvent molecules move inside the cell. This phenomenon is called endosmosis.
⦁ Exosmosis
When a cell is placed in a hypertonic solution, the solute concentration is higher on the outside of the cell, therefore the solvent moves outside the cell, making the cell go flaccid. This phenomenon is known as Exosmosis.
Effects of Osmosis in Science
Osmosis affects animal and plant cells differently because their cells react differently in different osmotic solutions. When you place an animal cell in a hypotonic solution, it will start lysing and burst open. Whereas a plant cell will not be affected at all. In fact, a hypotonic solution is ideal for plant cells.
Plant cells have thick walls and require a lot of water to survive, which is why, the plant cells will not burst, making the hypotonic solution perfect for them.
However, an isotonic solution is not good for plant cells, but ideal for animal cells. In this solution, the plant cells no longer remain turgid as the solvent seeps out of them and begin to droop.
However, when the solute concentration is equal on both sides of the cell, the animal cell thrives in it, this osmotic flow can be reversed as well as stopped by applying external pressure to the sides of the solute.
Some of this might be a bit confusing and may not initially make sense, but keep on reading and things will start becoming more digestible.
Significance of Osmosis in Science
Osmosis is one of the most important phenomena of biology. Without osmosis, there are many biological systems that can not exist. Let’s see the importance of osmosis in animal and plant bodies:
⦁ Osmosis is a big part of nutritional transport in plants and animal cells. It influences the release of metabolic waste from the cell and takes in the nutrition needed by the cells.
⦁ It affects the turgidity and flaccidity of the cells.
⦁ In all living organisms, osmosis stabilizes the internal environment by controlling the balance between the intercellular fluid levels and water.
⦁ Osmosis also plays a big part in the diffusion of water from one cell to another.
⦁ In plants, osmosis is responsible for the water absorption from the soil and transporting it to the aerial parts of the plant.
⦁ Osmosis also maintains the water content in plants despite the constant transpiration.
⦁ Osmosis also induces cell turgor in plants which affects the movement of plants and their parts.
⦁ When there are drought conditions, high osmotic pressures protect the plants against injuries.
⦁ In animal cells, osmosis plays a huge part in the absorption of water in blood cells from the intestines.
Some Osmotic Examples
Now, as you might have established, osmosis is one of the most important functions for animals and plants. So, here are some examples of osmosis:
⦁ Absorption of Water from the Soil
The plant roots have a higher solute concentration, which is where osmosis works its magic and the water under the soil, migrates through the roots into the plant and travels upwards against the gravity.
⦁ Plant Guard Cells
Transpiration occurs through the process of osmosis. Then the guard cells are filled up with water, they become turgid which allows the stomata to open up and transpiration takes place.
⦁ Osmosis in Fish
Freshwater fish cannot survive in saltwater and similarly, saltwater fishes cannot survive in freshwater. When a saltwater fish or a freshwater fish is placed in water that has different concentrations of salt, the fishes die due to the entry and exit of water through their cells.
⦁ Fingers in Water for a Long Time
When you place your fingers in water for long periods of time, they become pruney (you observe wrinkles on them). This is because the water flows inside the skin cells after they have been in the water for a long time. The quicker your finger prunes, the quicker your body is absorbing water.
Wrapping Up
Osmosis is an important aspect of life because of many reasons. It helps in the transportation of all things to and from the cell. From nutrients to water and many other solutes, osmosis keeps the cells safe and alive. It only allows the important things to enter and leave the cell, leaving out any harmful substances from entering or important substances from leaving it!